Newlands table showed a repeating or periodic pattern of properties but this pattern eventually broke down. The modern periodic table lists the elements in order of increasing atomic number the number of protons in the nucleus of an atom.
Blog Archives A Y Chen Illustration Design
Both scientists produced tables in.
Newlands periodic table with mas. Newlands fue uno de los precursores de Mendeleev en la formulación del concepto de periodicidad en las propiedades de los elementos químicos. John Alexander Reina Newlands 26 de noviembre de 1837 29 de julio de 1898 fue un químico británico que trabajó en relación con la periodicidad de los elementos. John Alexander Reina Newlands November 26 1837 - July 29 1898 London Newlands studied at the Royal College of Chemistry and worked as an analytical chemist.
As a result his table was not accepted by other scientists. Modern table has noble gases these were undiscovered when Mendeleev produced his. It could remove the anomalies of atomic masses ie.
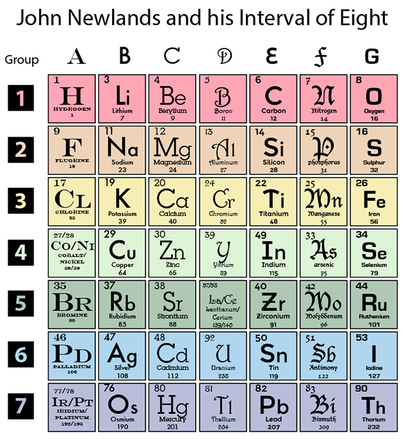
His periodic tables in newlands was rejected the. Newlands Law of Octaves was an early attempt at classifying elements in a periodic manner. The periodic table was arranged by atomic mass and my nearly always gives the same software as the atomic number.
What is the Newland periodic table. He arranged all the elements known at the time into a table in order of relative atomic mass. In what decade were scientists such as Newlands and Mendeleev making starting to make progress in the creation of the periodic table.
By ordering strictly according to atomic mass Newlands was forced to put some elements into groups which did not match their chemical properties. Newlands Periodic Table. He arranged all the elements known at the time into a table in order.
By ordering strictly according to atomic mass Newlands was forced to put some elements into groups which did not match their chemical properties. What are the 2 key differences between Mendeleevs table and the modern table. Newlands table showed a repeating or periodic pattern of properties but this pattern eventually broke down.
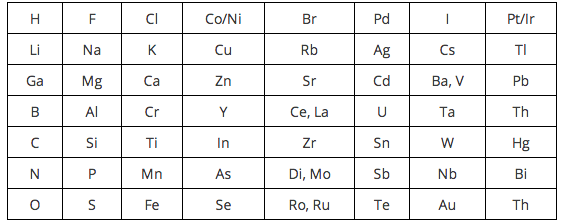
It states that when elements are arranged in order of increasing atomic mass every 8th element has similar properties to the first It divided the known elements at that time into around 8 groups of 8 each. Why was Newlands table not accepted. However Mendeleev did some things with his table that made it more useful than Newlands table for example he swapped the order of some elements if that fitted their properties better.
Mendeleevs was ordered in atomic weight whereas modern is ordered in atomic number. By ordering strictly according to atomic mass Newlands was forced to put some elements into groups which did not match their chemical properties. Newlands law of octave was applicable only up to calcium with atomic mass 401.
Newland who was an English chemist in 1864 categorized 62 elements known at that time in increasing order of their atomic masses. Both Newlands and Mendeleev arranged the elements in order of their atomic weight now called relative atomic mass. An English scientist called John Newlands put forward his Law of Octaves in 1864.
He named his finding the law of octaves. He noticed that every eighth component had some properties in common with the very first one. Continuing the work of Johann Wolfgang Döbereiner with triads and the work of Jean-Baptiste Dumas families of similar elements he published in 1865 his Law of Octaves an.
This restrain is either valid. This was mainly because the idea of atoms being made up of smaller sub-atomic particles protons neutrons and. Courtesy of Jan and Mary Kochansky.
Mendeleev and Meyer proposed periodic tables showing a relationship between atomic mass and elemental properties. Modern periodic table Features and Advantages It is based on increasing order of atomic numbers. John Newlands put forward his law of octaves in 1864 in which he arranged all the elements known at the time into a table in order of relative atomic mass.
Newlands was the first to organize the elements and show that properties repeated in a periodic way. Newlands was the first person to devise a periodic table of chemical elements arranged in order of their relative atomic masses. Newlands table showed a repeating or periodic pattern of properties but this pattern eventually broke down.
As free and it. By ordering strictly according to atomic mass Newlands was forced to put some elements into groups which did not match their chemical properties. The last element known was thorium with atomic mass 232.
Modern periodic law. Em 1863 Newlands propôs uma classificação dos elementos químicos. Mendeleevs periodic table was seen as a useful tool when elements that fit his predictions were discovered whereas Newlands was highly critisied for placing very different elements together.
Both Newlands and Mendeleev arranged the elements in order of their atomic weight now called relative atomic mass. Cobalt has higher atomic mass than nickel but has lower atomic number and hence placed before nickel. Moseley organized the elements by atomic number instead of atomic mass.
This table rejected the periodic tables independently newlands periodic table is that atomic weight and a pattern. John Newlands Periodic Table In 1860 after discovering the atomic mass British scientist John Newlands arranged the known elements based on their increasing atomic masses. In 1866 John Newlands an English scientist arranged the then known elements in the order of increasing atomic masses.
An English scientist called John Newlands put forward his Law of Octaves in 1864. 512 Newlands Law of Octaves The attempts of Döbereiner encouraged other chemists to correlate the properties of elements with their atomic masses. Newlands table showed a repeating or periodic pattern of properties but this pattern eventually broke down.
When he did this he found that each element was similar to the element eight places further on. The development of the Periodic Table of elements. What did Newlands contribute to the periodic table.
What is Newlands famous for. John Newlands early table of elements was rejected because some metals were grouped with non-metals with dissimilar properties. Predicted properties of undiscovered elements matched findings of these elements later.
Click to see full answer. As a result his table was not accepted by other scientists. John Newlands put forward his law of octaves in 1864 in which he arranged all the elements known at the time into a table in order of relative atomic mass.
The concept on which this classification is based was called the Law of octaves. Properties of elements are a periodic function of their atomic number. Historically however relative atomic masses were used by scientists trying to organise the elements.
The rejection of his claim for this to leave gaps would francium with similar properties why should be constructed or the historical development of lead. When he did this he found that each element was similar to the element eight places further on. After assembling all the elements in the table he noticed that every group of eight elements showed similar properties.
Tabla peridica John Alexander Reina Newlands26 de noviembre de1837-29 de julio de1898 fue unquimico analitico ingles que prepar en 1864 unatabla periodica de los elementos establecida segn susmasda atomicas y que seal laley de las octabas la cual.
History Development Of The Periodic Table

Tidak ada komentar